Coupling Electrochemical Oxidation with Aerobic Biodegradation for Water Contaminants
- Magneli Materials

- Aug 26, 2019
- 1 min read
Updated: Aug 28, 2019
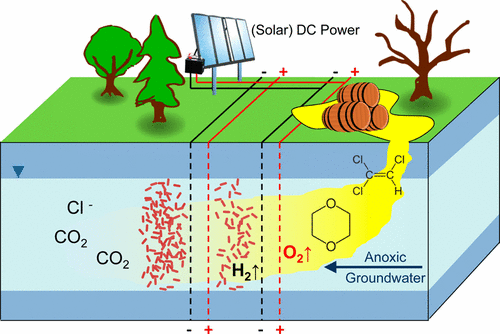
Synergistic Treatment of Mixed 1,4-Dioxane and Chlorinated Solvent Contaminations by Coupling Electrochemical Oxidation with Aerobic Biodegradation
"Biodegradation of the persistent groundwater contaminant 1,4-dioxane is often hindered by the absence of dissolved oxygen and the co-occurrence of inhibiting chlorinated solvents. Using flow-through electrolytic reactors equipped with Ti/IrO2–Ta2O5 mesh electrodes, we show that combining electrochemical oxidation with aerobic biodegradation produces an overadditive treatment effect for degrading 1,4-dioxane. In reactors bioaugmented by Pseudonocardia dioxanivorans CB1190 with 3.0 V applied, 1,4-dioxane was oxidized 2.5 times faster than in bioaugmented control reactors without an applied potential, and 12 times faster than by abiotic electrolysis only. Quantitative polymerase chain reaction analyses of CB1190 abundance, oxidation–reduction potential, and dissolved oxygen measurements indicated that microbial growth was promoted by anodic oxygen-generating reactions. At a higher potential of 8.0 V, however, the cell abundance near the anode was diminished, likely due to unfavorable pH and/or redox conditions. When coupled to electrolysis, biodegradation of 1,4-dioxane was sustained even in the presence of the common co-contaminant trichloroethene in the influent. Our findings demonstrate that combining electrolytic treatment with aerobic biodegradation may be a promising synergistic approach for the treatment of mixed contaminants."
An excerpt of an article published in Environmental Science and Technology, by Jeramy R. Jasmann, Phillip B. Gedalanga, Thomas Borch, Shaily Mahendra & Jens Blotevogel.
Read the full article here: https://pubs.acs.org/doi/full/10.1021/acs.est.7b03134


Comments